Site brought to you by PolySciTech
Acronym:PLGA-PEG-Mal
Name:Poly(lactic-co-glycolide)-b-Poly(ethylene glycol)-Maleimide endcap
Catalog Number:AI020
Special Property:Allows for generation of custom-targeted ligands
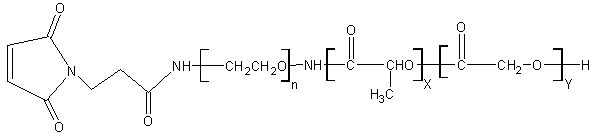
How Used:
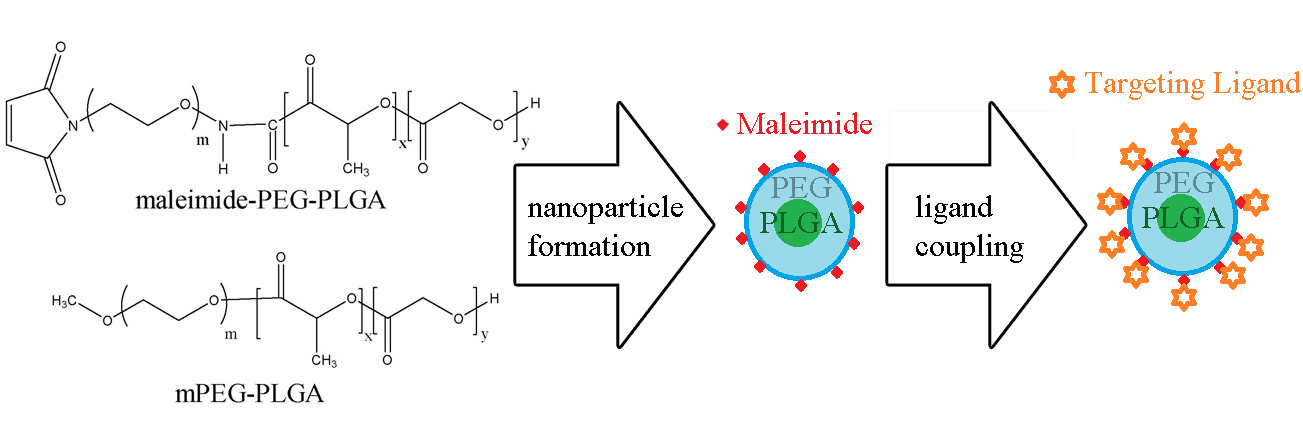
The diblock nature of the PLGA-PEG-Maleimide allows for the formation of micelles, either by traditional emulsion techniques or modern microfluidic methods. Since 100% labeling of the micelle is typically unnecessary to achieve targeting, the PLGA-PEG-Mal is physically mixed with PLGA-PEG-Methoxy (PEG diblock copolymers). Methoxy provides only a chemically inert endcap with no conjugation properties.
It has been reported [1] that a mixture of 9:1 (weight methoxy-PEG-PLGA:weight Maleimide-PEG-PLGA) is sufficient for targeting. The micelles, formed with a hydrophobic drug inside, are reactive toward thiol units present on cysteine amino acids. That allows for conjugation of peptide, protein, or other thiol-containing components to the exterior of the micelle, thus decorating it with the ligand. Once decorated the micelles actively bind to the target of the ligand, typically a cell-surface receptor. And allow for more direct methods of drug delivery than by passive targeting alone. Depending on size, the micelles may also be eligible for enhanced permeation and retention (EPR) of tumor cells, in addition to direct binding.
Example Protocols
Dissolution
Typically the diblock copolymer is dissolved in a suitable organic solvent along with the drug to be delivered. Solvents for this include halogenated solvents (dichloromethane, chloroform), ketone (acetone), and nitrile-based (acetonitrile) as well as others (dimethylsulfoxide, dimethylformamide) [2]. At this step, avoid any solvents containing thiol units as they will react prematurely with the maleimide endcap. At the dissolution stage a variety of organic soluble drugs can be incorporated, such as anti-cancer compounds (paclitaxel, doxorubicin), immune inhibitors (everolimus), or hydrophobic fluorescent dyes, to ensure drug delivery and/or for theranostic use (coumarin-6) [1,3].
W/O/W Emulsion
Example
Probe sonicator method (From Luo, 2010 [3])
- Dissolve desired ratio of mPEG–PLGA and maleimide–PEG–PLGA in dichloromethane (30 mg total mass/1 mL). To label with coumarin-6 add 15 µL of coumarin-6 (1 mg/ml) solution to this dissolution.
- Emulsify 50 µL of water with continuous sonication (30 s) on ice using a probe sonicator at 160 W into the DCM solution containing mPEG–PLGA and maleimide–PEG–PLGA.
- Take this primary emulsion and sonicate at 160 W (30 s) on ice into 2 ml of 1% sodium cholate aqueous solution.
- The formed w/o/w emulsion is then diluted into 38 ml of a 0.5% sodium cholate aqueous solution under rapid magnetic stirring for 10 minutes.
- After 10 minutes, put solution in rotovap and heat to 40° C under vacuum to remove DCM.
- Collect nanoparticles (NPs) by centrifugation at 14,000 rpm at 4° C for 45 minutes.
- Discard supernatant, and resuspend NP’s in minimal volume of water and store at 4° C until ready to use.
Example: Nanoparticle Purification [1]
- Take the emulsion-formed nanoparticle suspension and subject it to a 1.5 x 20 cm sepharose CL-4B column.
- Elute with 0.05 M HEPES buffer (containing 0.15 M NaCl, pH 7.0) to remove any un-entrapped coumarin-6.
Protein-Ligand Maleimide-Thiol Reaction
Example: Conjugation to LYP1 (a 9-amino-acid cyclic peptide identified on MDA-MB-435 human carcinoma tumors) [3]
- LYP1 (synthesized separately) is mixed with nanoparticles at a peptide:maleimide ratio of 1.3:1 in PBS (pH 7.0).
- Reaction is performed overnight at room temperature with gentle agitation.
- The following day the NP’s are concentrated by centrifuging at 14,000 rpm at 4° C for 45 minutes.
- LYP1 is cyclicized by mixing NP’s with 0.2 mol/L citric acid at a concentration of 1 mg/mL.
- One-tenth volume of 1 mol/L hydrochloride is added to this mixture.
- Immediately 5 mmol iodic methanol solution is added at a ratio of 1:1 peptide:iodine.
- 1 M ascorbic acid is added dropwise until color becomes clear.
- NP’s are collected at 14,000 rpm at 4° C for 45 minutes.
Example: Conjugation to Pep TGN (a 12-amino-acid-peptide which functions to aid crossing the blood-brain-barrier (BBB) [1]
- Reaction is performed by coupling at room temperature for 8 hours between nanoparticles and Pep TGN at 1:3 or 1:1 mole ratio.
- The resultant nanoparticles purified by passing through a 1.5 x 20 cm sepharose CL-4B column to remove unconjugated proteins.
Protein-Ligand Reaction Confirmation
Example: Confirmation of LYP1 [3]
- Form ligand conjugated LYP1 NP’s as described above.
- As a control form NP’s with no LYP1.
- Incubate both sets with FITC at room temperature and quantify fluorescence by spectrofluorometer.
Post conjugation, the application of the ligand nanoparticles to appropriate cell-cultures and animal models (such as xenografted tumor-bearing mice) can be utilized to validate the overall effectiveness of the targeting system. This is shown in the following example protocols.
Example: In-Vitro Cell Culture PLGA-PEG-LYP1 [3]
- Incubate BxPC-3 (cancer) cells in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS) at 37° C in a 5% CO2 humidified incubator. Media changed every 48 hours.
- Passage the cells using EDTA-trypsin when confluent.
- Treat cells cultured with addition of 0.5 mg/ml of coumarin-6 loaded LYP-NP’s and incubate for 24 hours. Additionally utilize positive and negative controls (blank NPs, NP’s of only mPEG-PLGA, cells pretreated with interfering targeting ligand etc.) per experimental design.
- Image cultured cells using microscopy for qualititative analysis and utilize FACScan Flow Cytometry for quantitative analysis of coumarin-6 uptake.
Example: Mouse-Model PLGA-PEG-LYP1 [3]
- Inject subcutaneously into nude BALB/c mice 1x106 BxPC-3 cancer cells via nail pad to induce metastatic tumor model. Allow to progress for 3 weeks.
- Introduce coumarin-6 loaded NP’s with or (as a control) without LYP1 targeting ligand via nail pad at a dose of 60 mg/kg.
- Twelve hours later sacrifice animals and harvest tissues.
- Image all relevant organs (liver, spleen, lungs, heart, brain, kidneys, etc.) and tumor tissue using a living imaging system equipped with a UV filter. To determine relative uptake of nanoparticles into each region.
Helpful Links
The following full-text links utilize PCL-PEG-Maleimide which is functionally similar
Gindy et. al. 2008. Extremely well-written paper regarding Bovine Serum Albumin (BSA) conjugation utilizing PCL-PEG-Maleimide (functionally similar to PLGA-PEG-Mal). Available in full text (PDF).
Alexis, et. al. 2008, "HER‐2‐Targeted Nanoparticle–Affibody Bioconjugates for Cancer Therapy" (PDF).
Lai et. al. 2010, "AS1411 Aptamer-Conjugated Polymeric Micelle for Targetable Cancer Therapy".
Tai, et. al. 2010, The role of HER2 in cancer therapy and targeted drug delivery.
References
[1] Jingwei Li, Liang Feng, Li Fan, Yuan Zha, Liangran Guo, Qizhi Zhang, Jun Chen, Zhiqing Pang, Yuchen Wang, Xinguo Jiang, Victor C. Yang, Longping Wen, Targeting the brain with PEG–PLGA nanoparticles modified with phage-displayed peptides, Biomaterials, Volume 32, Issue 21, July 2011, Pages 4943-4950, ISSN 0142-9612, 10.1016/j.biomaterials.2011.03.031. Keywords: Phage display; Blood-brain barrier (BBB); Brain delivery; Biodegradable nanoparticle.
[2] Y.-Y. Huang, T.-W. Chung, T.-w. Tzeng, "Drug release from PLA/PEG microparticulates";, International Journal of Pharmaceutics 156(1) (1997) 9-15.
[3] Guopei Luo, Xianjun Yu, Chen Jin, Feng Yang, Deliang Fu, Jiang Long, Jin Xu, Changyou Zhan, Weiyue Lu, LyP-1-conjugated nanoparticles for targeting drug delivery to lymphatic metastatic tumors, International Journal of Pharmaceutics, Volume 385, Issues 1–2, 29 January 2010, Pages 150-156, ISSN 0378-5173, 10.1016/j.ijpharm.2009.10.014. Keywords: PLGA; PEG; Nanoparticle; Targeting; Drug delivery; Tumor; Lymph metastasis.
